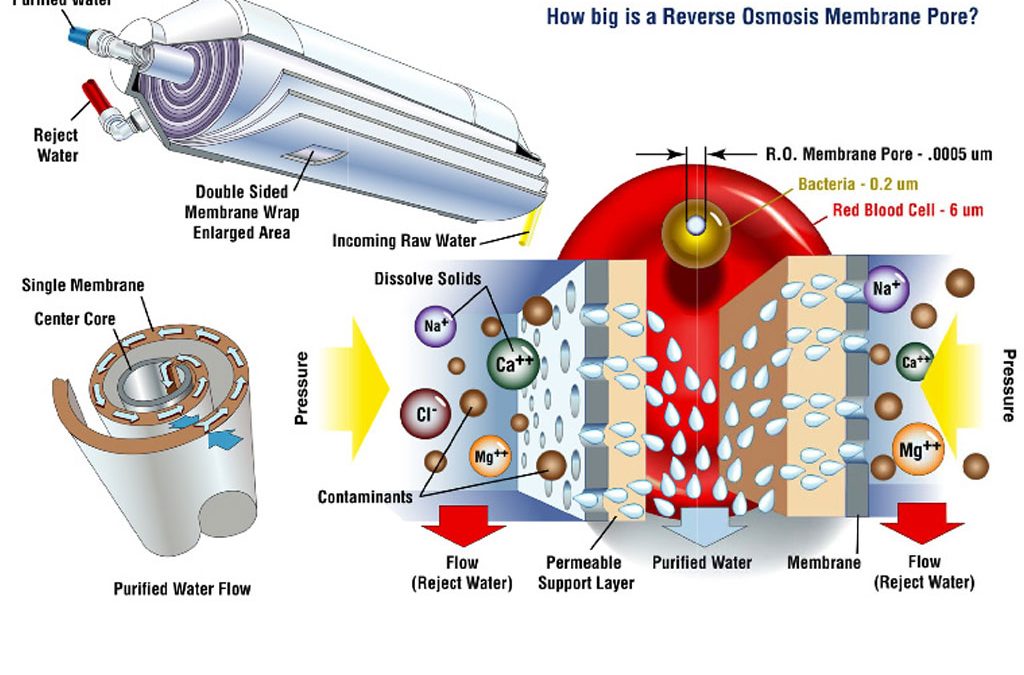
Reverse Osmosis also known as “RO”
Reverse osmosis is a process of separation that uses pressure to force a highly concentrated solute through a semi-permeable membrane to a region of lower solute concentration, which leaves the solute on one side and only the solvent on the other.
The image below depicts the process of Reverse Osmosis
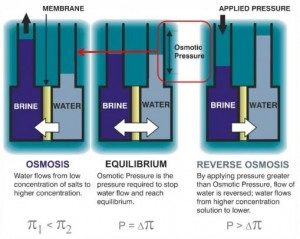
With the normal osmosis process, a liquid with two different concentrations will naturally move from an area of low solute concentration through a semi-permeable membrane to an area of high solute concentration to reach equilibrium. When two solutions are mixed together that contain different concentrations of a solute, the total amount of solutes in the solution will distribute equally. The semi-permeable membrane allows the solvent to move from one compartment to the other, but prevents the solute from doing the same. Osmosis is achieved when the solvent has moved from the areas of low solute concentration to the area of high solute concentration.
The method in which reverse osmosis works is that it uses the opposite process by which the normal osmosis process flows. With reverse osmosis, the same concept is applied with that of normal osmosis, but in this case, pressure is applied to the side of high solute concentration so that force influences the movement of the water as well as the normal osmotic pressure in osmosis. In order for reverse osmosis to occur, the pressure of the feed water must exceed that of the osmotic pressure.
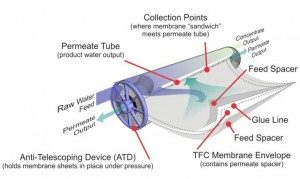
At the heart of the reverse osmosis process is the membrane used to filter the concentrated solution. There are various types of membranes used in reverse osmosis, two of which are cellulose triacetate membranes for use with heavily chlorinated water, and thin film composite membranes for use with waters with high TDS (total dissolved solids). Thin film composite membranes are typically constructed using numerous layers of materials bonded together and wound around a spiral core tube. One of the layers of film used is polyamide, which is permeable to water and impermeable to other impurities like salt ions. These types of membranes are typically used in water purification or water desalination systems.
The rate of liquid passage through the membrane during reverse osmosis depends on various factors such as pressure, concentration and the temperature of the molecules.
How reverse osmosis works within the membrane is by a process called cross flow filtration. The feed water is passed along the filter membrane at a positive pressure in relation to the permeate side. Water which is smaller than the membrane pore size passes through the membrane as processed permeate water; all other material is retained on the feed side. The permeate flows through the spiral tube of the membrane, while the concentrate does not and is retained. This allows the filter to operate continuously with the concentrate or waste water going to the drain.
Reverse osmosis works with a variety of applications and can be utilized in various ways. Reverse osmosis systems are typically found in households to improve the quality of water for drinking water. However, reverse osmosis is also widely used for commercial and industrial applications when the initial purchase and operating costs outweigh the negatives of water problems that one is experiencing. As of today, reverse osmosis is the most economical technology to purify water to high quality standards.

Hydroponic Nutrients

EC vs TDS

Organic Does Not Mean “No Pesticides”

How Plants Uptake Nutrients

What is Aquaponics
Trackbacks and pingbacks
No trackback or pingback available for this article.
0 comments
Leave a reply Delete Message
Articles
Featured
-
 Vegetable Formula single dose SetRegular Price $29.99
Vegetable Formula single dose SetRegular Price $29.99 -
 EzGro Quad Pot 25 PackRegular Price $274.99
EzGro Quad Pot 25 PackRegular Price $274.99 -
 Chemilizer InjectorRegular Price $349.99
Chemilizer InjectorRegular Price $349.99 -
 Rainwater Pressure Tank SystemRegular Price $449.00
Rainwater Pressure Tank SystemRegular Price $449.00 -
 EzGro Quad Pot 50 PackRegular Price $499.90
EzGro Quad Pot 50 PackRegular Price $499.90 -
 EzGro Precision Micro TrimmerRegular Price $11.99
EzGro Precision Micro TrimmerRegular Price $11.99 -
 Five Tower Deck GardenRegular Price $2,499.00
Five Tower Deck GardenRegular Price $2,499.00 -
 Cold Pressed Neem OilRegular Price $29.92 – $38.71
Cold Pressed Neem OilRegular Price $29.92 – $38.71 -
 Patio Garden Recharge KitRegular Price $49.00
Patio Garden Recharge KitRegular Price $49.00 -
 5000 Watt 48 Volt Power InverterRegular Price $899.00
5000 Watt 48 Volt Power InverterRegular Price $899.00









Best explanation of good water for hydroponic systems I've come upon.